Health
Japa: Council, nurses begin legal battle over new certification guidelines

Some Nurses in the country have sued the Nursing and Midwifery Council of Nigeria, and the Minister of Health among others over the new certificate verification guidelines.
The NMCN had on February 7, 2024, issued a circular revising the guidelines for requesting verification of certificates for nurses and midwives.
The council stated, among others, that applicants seeking verification of certificates from foreign nursing boards and councils must possess two years of post-qualification experience from the date of issuance of the permanent practising licence.
The new guidelines came into force on March 1, 2024.
But nurses and midwives, under the aegis of the National Association of Nigeria Nurses and Midwives, expressed concern that the NMCN’s revised guidelines for certificate verification were targeted at preventing them from going abroad in search of greener pastures.
They are particularly uncomfortable with the provision in the guidelines that a nurse seeking NMCN certification must have a minimum of two years post-qualification experience.
They are also opposed to the requirement that a nurse applying for NMCN’s certification must obtain a letter of good standing from the Chief Executive Officer of their place of work and the last training institution attended while the processing of application shall take a minimum of six months.
As a result of this, nurses in Abuja and Lagos protested to demand the reversal of the new guidelines.
Pushing their demands forward, some dissatisfied nurses on behalf of their colleagues dragged the Registrar, NMCN; the Coordinating Minister of Health and Social Welfare; Federal Ministry of Health; and the Attorney General of the Federation before the National Industrial Court in Abuja.
The complainants in the suit marked: NICN/ABJ/ 76/2024, are Desmond Aigbe; Kelvin Ossai; Catherine Olatunji-Kuyoro; Tamunoibi Berry; Osemwengie Osagie; Abiola Olaniyan; Idowu Olabode, and Olumide Olurankinse.
They are urging the court to restrain the defendants and their agents from implementing the NMCN circular pending the determination of the suit.
The nurses also urged the court to suspend the commencement of the new guidelines.
They want “an interlocutory order suspending the commencement of the 2nd defendant’s Revised Guidelines for Verification of Certificate(S) with the Nursing and Midwifery Council Of Nigeria, earlier proposed to take effect from the 7th of March, 2024 as indicated on the 2nd defendant’s circular dated 7th February, 2024, pending the hearing and determination of the claimants/applicants Originating Summons in this suit.”
They also want “an interlocutory order restraining the defendants, their partners, parastatals, subjects, counterparts and agents from taking any further step that may hinder, restrict, or infringe on the constitutional rights and freedom of nurses and midwives in Nigeria from emigrating to the country to seek better career opportunities and training abroad.”
At the proceedings on Wednesday, counsel for the complaints, Ode Evans, told the court that he had just received the preliminary objection filled by the first and second defendants.
He pleaded with the court to adjourn the matter to enable him to reply to their applications.
Evans said, “I confirmed the receipt of the application from the first and second defendants this morning. We shall be asking for a date to enable us to file our responses.”
Justice Osatohanmwen Obaseki-Osaghae adjourned the matter till May 20 for hearing.
She ordered that the hearing notice be served on the Federal Ministry of Health and the Attorney General of the Federation who had no legal representation in court.
Health
WHO calls for countries to address disruptions to TB services
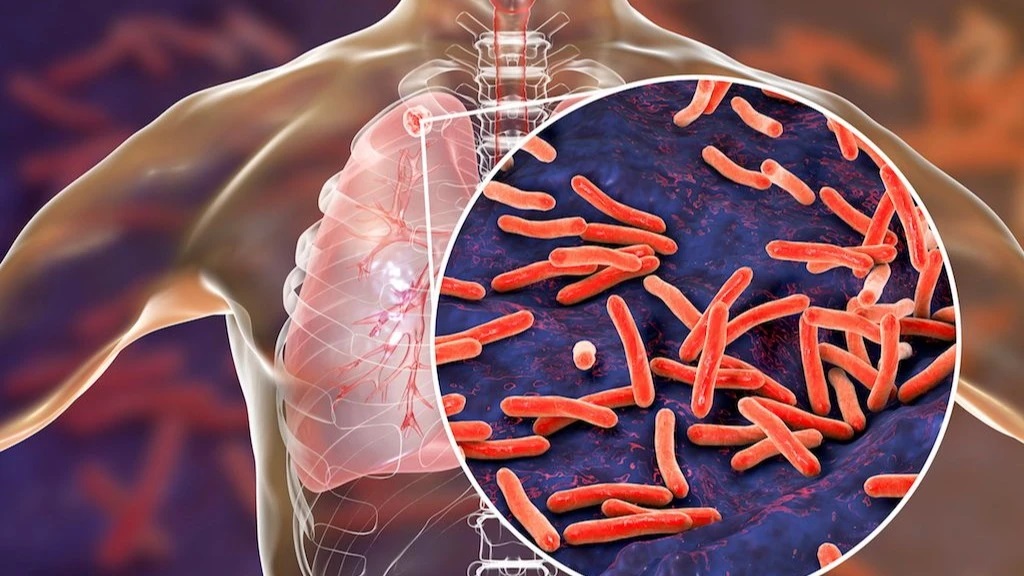
In the wake of massive cuts in US funding, the World Health Organization (WHO) today called on global health leaders, donors, and policymakers to protect and maintain tuberculosis (TB) care and support services around the world.
In a statement issued ahead of World Tuberculosis Day (March 24), the WHO said the “drastic and abrupt” cuts to global health funding threaten to reverse gains made in global efforts to combat TB, which remains the world’s deadliest infectious disease. Those efforts have saved an estimated 79 million lives worldwide since 2000, the organization said.
“The huge gains the world has made against TB over the past 20 years are now at risk as cuts to funding start to disrupt access to services for prevention, screening, and treatment for people with TB,” said WHO Director-General Tedros Adhanom Ghebreyesus, PhD. “But we cannot give up on the concrete commitments that world leaders made at the UN General Assembly just 18 months ago to accelerate work to end TB. WHO is committed to working with all donors, partners and affected countries to mitigate the impact of funding cuts and find innovative solutions.”
USAID cuts have crippled TB control efforts
While the statement does not specifically mention the US Agency for International Development (USAID), the Trump administration’s freeze of USAID funding, and the subsequent canceling of thousands of contracts issued by the agency, have left a gaping hole in funding for TB prevention, screening, and treatment services. The US government has been the leading bilateral donor to global TB control efforts, contributing $200 million to $250 million annually—roughly one quarter of international donor funding for the disease.
The WHO said 27 countries are facing crippling breakdowns in their TB response, with the biggest impact seen in high-TB burden countries in Africa, Southeast Asia, and the Western Pacific. Among the services that have been disrupted are diagnosis, active case finding, screening, and contact tracing, and those disruptions are resulting in delayed detection and treatment and increased transmission risk. Drug supply chains, laboratory services, and data and surveillance systems have also been undermined.
A recent update from StopTB Partnership, which works on TB response with more than 2,000 partners in 100 countries, provides some detail on the services affected by the USAID funding cuts. In Cambodia, active case finding has halted in half the country, resulting in 100,000 people missing TB screening and 10,000 cases of drug-susceptible (DS)-TB going undetected. In Kenya, sputum sample transport once supported by USAID has halted, affecting the diagnosis of DS- and drug-resistant (DR)-TB. In India, USAID-funded TB screening projects in vulnerable groups have stopped.
The huge gains the world has made against TB over the past 20 years are now at risk as cuts to funding start to disrupt access to services for prevention, screening, and treatment for people with TB.
Those are just three of dozens of examples. In a news release today, StopTB Partnership Executive Director Lucica Ditiu, MD, echoed Tedros’s call for action.
“People with TB need us,” Ditiu said. “We have to remain strong, and we can never ever give up the fight. Through innovative, global and national efforts and standing together, we will be able to achieve these targets of ensuring TB prevention, treatment, and care are accessible to all.”
TB was responsible for an estimated 1.25 million deaths in 2023, according to the WHO’s most recent annual report. An estimated 8.2 million people were newly diagnosed with the disease—the most cases in a year recorded by the WHO since it began global TB monitoring in 1995. High-burden TB countries have only recently begun to recover from the disruptions caused by the COVID-19 pandemic, which the WHO estimates resulted in 700,000 excess TB deaths.
Cuts exacerbate funding shortfalls
As the WHO notes, the funding cuts come amid what was already a shortfall in funding for global TB control efforts. In 2023, $5.7 billion was available for TB prevention, diagnostic, and treatment services in low- and middle-income countries, but that’s only 26% of the 2027 target goal of $22 billion. TB research is receiving just one fifth of its 2022 target of $5 billion. Cuts to US funding are only going to exacerbate the problem.
In a joint statement issued earlier this week, Tedros and the Civil Society Task Force on Tuberculosis called on countries to take urgent action to prevent any disruption to TB services, ensure domestic resources to sustain equitable and essential TB care, and safeguard essential TB drugs, diagnostics, care, and social protection coverage for TB patients. They also urged the establishment of national partner platforms that would bring together public and private sectors, civil society, nongovernmental organizations, professional societies, and donors to maintain momentum against TB in affected countries.
“This urgent call is timely and underscores the necessity of swift, decisive action to sustain global TB progress and prevent setbacks that could cost lives,” said Tereza Kasaeva, PhD, director of WHO’s Global Programme on TB and Lung Health, in today’s WHO news release.
Health
Women with VVF can have normal s3xual lives after treatment, say gynaecologists

By Francesca Hangeior
After proper surgical repair, women who have a vesicovaginal fistula, an abnormal opening between the bladder and the vaginal wall, leading to urine leakage and other complications, can have a pleasurable sexual life, gynaecologists have assured.
They advised such women to wait until 12 weeks after the surgery to allow complete healing and recovery before resuming sexual activities.
The maternal experts, however, stated that the return to normal sexual life depended on the delays before the repair was done and the scarred tissues that were formed, stating that some of the women might experience vagina tightness and pain during sex.
The fertility experts further emphasised the need for counselling and psychotherapy before resuming sexual activity, to ensure that the fear, anxiety, and emotional trauma related to sex and pregnancy were overcome.
According to the seasoned obstetric gynaecologists, women with repaired VVF, who desired more children, could become pregnant and have more babies.
However, they stressed that such delivery must be through a caesarean section and done in a conventional health facility.
VVF is a complication of obstructed labour during delivery. According to the United Nations Population Fund, VVF is a major public health problem with over two million cases, globally.
In Nigeria, there are about 150,000 cases with 12,000 new cases recorded every year.
Many women with VVF in Nigeria battle with stigma, leading to social ostracisation, abandonment, and psychological distress.
Also, a professor of Obstetrics and Gynaecology at the Usmanu Danfodiyo University, Abubakar Panti, stated that after the successful closure of the fistula and complete healing after surgery, normal sexual activity could be restored.
He further stated that to ensure proper healing and recovery time, the recommendation was to avoid sexual activity for at least three months, which is 12 weeks post-surgery to allow complete healing.
The don also stated that before resuming sexual activity, the strength of the pelvic floor must be assessed, as some women may experience vaginal tightness or weakness after prolonged fistula, which could affect sexual comfort.
To resolve this, Panti advised the women to do some pelvic floor exercises, noting that sometimes the help of a physiotherapist was needed.
“When women have lived with VVF for a long time, they know what caused it, they know it’s a pregnancy that caused it, so they may experience fear, anxiety or some form of emotional trauma related to sex, because they would think sex is what brought it in the first place, so they need a lot of counselling or therapy, in that instance psychotherapy may be beneficial.
“Some of these women used to have a lot of terrible experiences, sometimes they are abandoned by their husbands, divorced and other things, so they think every man may be like that.
“The last one probably would be the presence of scarring or vaginal shortening. If extensive damage occurred before repair, some women may have vaginal scarring and then sometimes there will be dryness of the vagina or reduced elasticity.
“Usually, the vagina distends to accommodate, irrespective of the size of the penis that comes into it, so if there is scarring, that distension will not be there, and there will be tightness, so this can also affect comfort during sex. Most of the time we just tell them to apply lubricants and sometimes medical interventions may help,” the fertility expert said.
Panti asserted that with successful surgery, proper healing and emotional support, many women regained a satisfying sexual life.
He advised the women to whenever they had concerns or difficulties, consult their gynaecologist, who would give them tips regarding the repair, sexual health and resuming sexual activity.
Panti emphasised the need for counselling the woman and her husband, “because it needs a lot of patience from the partner, that he has to start slow and of course, he has to listen to her or look at her body language if she’s in any discomfort or experiencing pain so that he can stop, rest and try again.”
The don advised the women to watch out for symptoms like leakage of urine and consult with the gynae if there’s discomfort during sex.
The consultant gynaecologist urged them to focus on their overall wellness by eating well and preventing urinary tract infections.
“With proper healing, patience and emotional support, most of them regain their full sexual life after VVF repair, the key is to slow down, communicate with your partner and seek medical advice where needed,” Panti said.
He further stated subsequent delivery after VVF must be by caesarean section, to prevent a reopening of the repair.
Health
Gynecologist’s caution pregnant women against Vaginal delivery after two CS

By Francesca Hangeior
Attempting labour and vaginal delivery after two previous caesarean sections could lead to a rupture of the uterine scar, resulting in severe bleeding and possible death of the expectant mother and her baby, maternal experts have warned.
The gynaecologists further noted that such deliveries posed risks of head compression and low oxygen supply and intake, leading to malformations.
The experts’ warning comes amid the stigma surrounding CS and the insistence of many Nigerian women who have previously undergone the procedure to attempt vaginal delivery in subsequent births.
Bleeding during and after delivery is a major cause of maternal mortality worldwide and in Nigeria.
In fact, it is the leading cause of maternal mortality in Nigeria, a country with one of the highest MMR in Africa.
The Nigeria Demographic and Health Survey, 2018, pegs the MMR at 512 deaths per 100,000 live births.
According to the World Health Organisation, every year, about 14 million women experience postpartum haemorrhage, resulting in about 70,000 maternal deaths globally.
A new study released by the WHO two weeks ago further revealed that severe heavy bleeding and hypertensive disorders like preeclampsia are the leading causes of maternal deaths globally.
It noted that the conditions were responsible for about 80,000 and 50,000 fatalities, respectively, in 2020, indicating that many women still lack access to lifesaving treatments and effective care during and after pregnancy and birth.
The experts urged expectant mothers to register for antenatal care and ensure delivery in healthcare facilities with skilled birth attendants to reduce risks and ensure optimum care for both mother and child.
A recent study on “Trial of labour following two previous caesarean sections – A UK cohort study” concluded that women considering a trial of labour following two caesarean sections had an increased risk of endometritis (infection of the inner lining of the uterus), sepsis and adverse neonatal outcome.
Providing expert insight into the matter, a Professor of Obstetrics Gynaecology at the College of Health Science, University of Uyo, Akwa Ibom State, Aniekan Abasiattai, explained that after a woman undergoes CS, the cut, after healing, forms a scar.
The don added that a woman who has undergone CS twice and in subsequent pregnancy attempts to go into labour and vaginal delivery, had an increased risk of tearing the scar, leading to bleeding.
He further noted that although women who have had one caesarean delivery could be allowed to attempt a vaginal delivery, it was done in specialised units and with close monitoring.
“Now, after two caesarean sections, because of the increased risk of rupture of the scar, which is much more than that of a previous caesarean delivery, in this environment, we usually do not allow our patients to attempt a vaginal delivery after two previous caesarean sections. That’s the standard in this country.
“I’m aware that there are varying publications of successful vaginal deliveries after two previous caesarean sections, both in the developed world, foreign literature, and even among a few of our colleagues, but we usually do not, that is not the accepted practice, basically, because of the increased risk of infection following surgical procedures, deliveries, whether vaginal or caesarean delivery,” Abasiattai said.
Speaking on the impact on the babies, the gynaecologist said, “When the uterus ruptures, it cuts off and the baby becomes affected directly. Low oxygen transfer, hypoxia sets in, and the rate of death or foetal mortality is quite high. Even in some instances, more than 50 per cent following rupture of the scarred uterus.
“So apart from the fact that the woman can have complications from excessive haemorrhage from the torn uterus, the baby, in a significant proportion of cases, dies inside the uterus. Unless surgical intervention is done promptly to arrest the ongoing haemorrhage, repair or stop the bleeding and then deliver the baby.”
The researcher on Community Obstetrics, Fetomaternal Medicine and Reproductive Health urged women who have had previous CS to refrain from having their next delivery at unconventional health facilities, stating that they had an increased risk of a ruptured uterus, among other complications.
Also, a Professor of Obstetrics and Gynaecology at the Obafemi Awolowo University, Ile-Ife, Osun State, Ernest Orji, stated that it was not safe for a woman to attempt labour and vaginal delivery after having two caesarean sections.
He explained, “It’s risky because the womb has been cut two times, and they say you don’t use a wounded soldier to go to battle. The chances of tearing or rupturing during labour are high.
“That’s why we tell women that if you have had caesarean section two times it is not safe to allow you to go into labour because during labour, the womb will be contracting and pushing and so the risk of the womb rupturing and the mother and baby dying is very high.”
The don stated that although there were reports of some women who despite having a history of two CS, tried vaginal delivery and went unscathed, such procedure was not advisable.
Speaking on the implications for the mother and baby, Orji said, “The first danger is that the womb can tear and when that happens, the baby may die depending on the site of the tear. The tear would make the woman start bleeding and when the bleeding is too much, she can bleed and die.
“When the woman is bleeding and is rushed to the hospital, sometimes, by the time they come to the hospital, it may be too late and you will have to remove the womb.
“So, apart from the risk that the woman may die, another risk is the fact that you may have to remove the womb because the womb may be so damaged that it can no longer be repaired.”
The researcher on Reproductive and Feto-maternal health further stated that the babies born through such a process may have their heads compressed, which could affect the babies’ brain and intellectual performance later in life.
-

 News12 hours ago
News12 hours agoOERAF held memorial lecture on conflict resolution, security/safety of community in Nigeria
-

 News21 hours ago
News21 hours agoJust in: Founder of Diamond Bank and ex-chairman of MTN, Paschal Dozie is dead
-

 News17 hours ago
News17 hours agoTRADE WAR! U.S. angry over Nigeria’s import ban on 25 products
-

 News22 hours ago
News22 hours agoNaira Nosedives Against Dollar
-

 Sports21 hours ago
Sports21 hours agoReal Madrid keeping tabs on Victor Osimhen
-

 News22 hours ago
News22 hours ago2Baba’s Lover Natasha Osawaru Fired As Edo Assembly Minority Leader
-

 News17 hours ago
News17 hours agoINTERVIEW: Introduction of Child Rights Curriculum In Nigerian Universities Will Take CRA to Families – Dr Obiorah Edogor
-

 News21 hours ago
News21 hours agoOERAF Executive Director Dr Akpodiete, Held Memorial lecture on Essence and benefits of health insurance+Photos





